Metrics are instrumental in assessing the level of performance in all aspects of life, be it engineering, sport or business – tensile strength, lap times, share price; the list is endless.
Cleanroom technology for life science buildings is an area that demands particularly robust performance validation; every research establishment or pharmaceutical manufacturer’s licence application must be supported by validated metrics.
The data delivers assurance that the correct levels of operator, product and environmental protection are being delivered by the ventilation systems providing clean, particle- and pathogen-free air to the critical space.
With the ongoing Covid pandemic, we believe there is an opportunity for a technology transfer from the established cleanroom design and validation principles to mechanically ventilated spaces in commercial buildings.
Covid has highlighted the importance of ventilation in the mitigation of risk from airborne infection in mechanically ventilated spaces. It’s essential that equipment that claims to clean air has been tested and validated, as CIBSE emphasised in its 2020 Covid-19 guidance.1 It notes that there was a lack of data supporting the claims of a new generation of air-cleaning systems and technologies.
We set out to demonstrate and measure the potential benefits of the technology transfer of the principle of clean-air technologies from the life science sector to offices and other commercial buildings.
For many decades, the life sciences industry has employed sophisticated ventilation systems and contamination-control protocols, with the efficacy validated under stringent regulatory guidance. Pharmaceutical products are manufactured in cleanroom environments that control levels of airborne particulate for product protection.
We believe the tested system – with its associated refined, disciplined and accountable processes – provides the framework to improve and quantify workplace ventilation systems.
What is a cleanroom?
A cleanroom is a room with environmental control of particulate contamination, temperature and humidity, constructed in such a way as to minimise the introduction, generation and retention of particles inside the cleanroom.
There is an opportunity for a technology transfer from the established cleanroom design and validation principles to mechanically ventilated spaces in commercial buildings
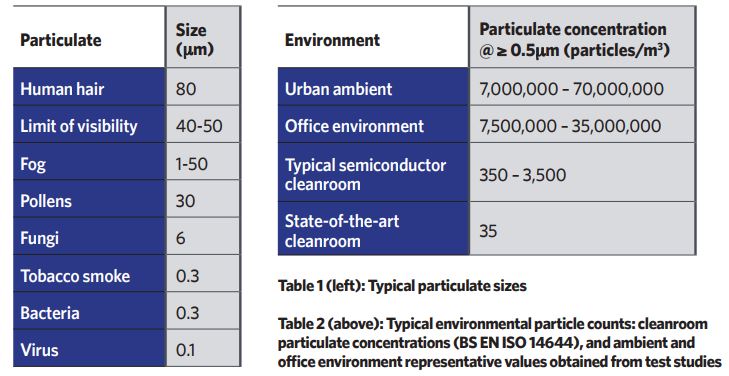
All cleanrooms are classified based on the number of particles in a cubic metre of sampled air, down to the sub-micron scale, as per BS EN ISO 14644 Cleanrooms and associated controlled environments. Tables 1 and 2 provide some context, demonstrating the required reduction in airborne contamination.
This level of cleanliness is reached by employing a range of technologies and procedures, with the key methods being:
- HEPA filtered supply air
- Positive room air-pressure cascades
- High air-change rates
- Room finishes
- Gowning of operatives
- Cleaning regime.
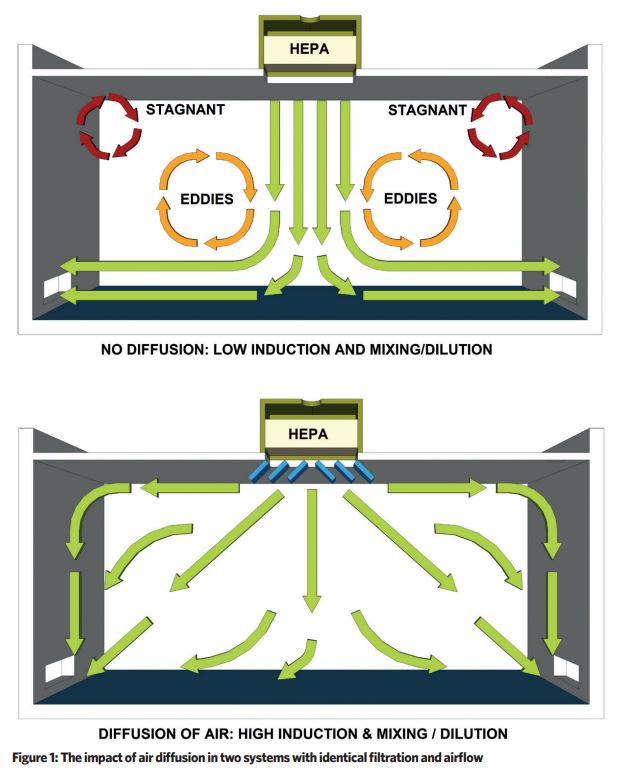
The key principle is to achieve top-down air diffusion ventilation, with sufficient velocity and distribution to keep particles buoyant, so that they may be driven to air extraction/return grilles and removed from the air ventilation system by HEPA filtration.
Ventilation air diffusion patterns are usually a compromise between the ideal arrangement of high-level laminar supply and low-level extract, and the practical arrangement of the space to accommodate equipment and processes.
Figure 1 illustrates the impact of air diffusion, and the difference between two systems with identical filtration and airflow volume flowrates.
Pre-emptive techniques such as gowning protocols or micron-scale cleaning are extremely challenging to practically implement in a typical workplace environment. However, proactive methods – such as HEPA filtration, air diffusion technology and positive pressurisation – require minimal adaptation. These tests measured the impact that proactive methods could have on the reduction of airborne particulate and, thereby, the associated risk of airborne infection of occupants
Constructing a full-scale mock-up
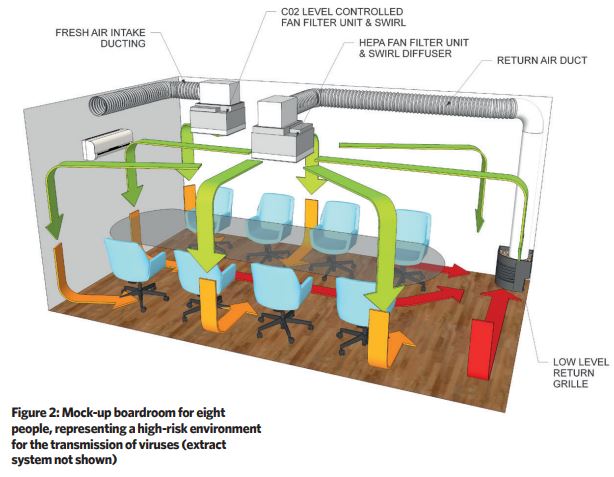
A boardroom measuring 3.3m x 5.5m x 2.5m was selected to act as the test cell for a cleanroom-type ventilation installation. This room accommodates eight occupants in a relatively tight space, which provides a good representation of a high-risk environment for the transmission of viruses (see Figure 2).
The boardroom was fitted with two 600mm x 600mm H14 (99.995% effective against most penetrating particle size) HEPA fan filter units (FFUs) with bespoke swirl diffusers, each diffusing 540m3.h-1 of clean air into the space.
One unit was connected to a low-level return air grille, designed to blend aesthetically with the office environment, which ran at a constant rate to manage the ‘base load’ of particulate contamination generated by the occupants. The second unit was ducted to a fresh air supply and was controlled from a signal provided by a wall-mounted CO2 sensor.
Increased energy consumption is an unavoidable consequence of filtering air because of the resistance generated by the filter media and the corresponding load on the fan. So it is important to keep the volume of air filtration to the minimum required to mitigate risk rather than to operate at 100% duty, 24/7.
Real-time particle counting was investigated for controlling the fan speed, but these counts are not a good analogue for risk, as particles will be generated by the surfaces and objects within the space.
Elevated CO2 concentration corresponds very well with elevated human activity – this was employed as a proxy for potential air contamination in these tests. This second unit remains ‘idle’ until there is a demand for ventilation air that is triggered by rising CO2 levels – the unit’s fan speed modulates to manage the associated airborne risks presented by the human activity in the room.
The first unit provides approximately 12 recirculation air changes per hour, and the CO2-activated fan will double this at peak occupant demand with ventilation air.
Comparative testing of solutions
To assess the efficacy of the boardroom system, a test – based on cleanroom industry practices – was devised to compare its performance against a commercially available mobile unit.
The space was challenged with particulates at a concentration of more than 100,000 per m3. A particle counter took cumulative counts every minute to determine the rate at which each ventilation system cleaned down the space.
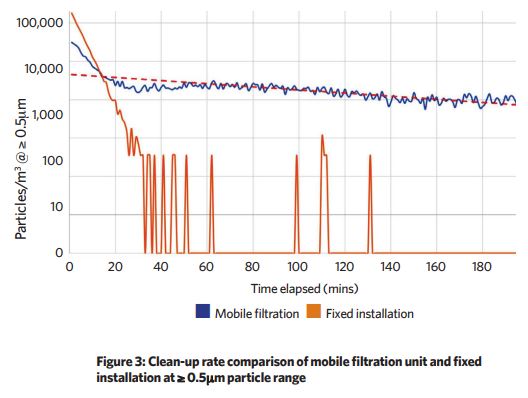
The top-down, highly-diffused air supply system cleaned down to zero detectable particles after 30 minutes, with occasional trace values logged thereafter – this was achieved running a single recirculating fan, with the fresh air system disabled.
A mobile unit, circulating the same volume of air across the same grade filter, achieved a much slower clean-up rate, achieving a particle volume of around 1,000 per m3 after three hours.
These results show a significant improvement in clean-up rates through the use of a top-down, highly diffused air supply.
Research carried out using the test cell indicates that trapping particulates and managing energy consumption on a ‘matched to demand’ basis, using CO2 monitors, provides an acceptable trade-off between energy use and air quality. Outputs from the work can inform a number of design parameters that go beyond those discussed in this article – see panel ‘Design considerations’ (below).
One consequence of this research is real-time monitoring of particles in the cleanroom sector. Currently, HEPA filters may be checked once every six to 12 months, but real-time monitoring using a particle counter will allow operators to see when filters need changing.
CIBSE’s call to action to effectively meet, and evidence, the needs of air cleaning not only led us to understand the efficacy of diffuse air systems in commercial settings, but also enabled knowledge transfer back to the cleanroom sector, which will potentially save time and money in the maintenance of life science laboratories.
DESIGN CONSIDERATIONS
- Employ inline or terminal HEPA filters
- Utilise suitably sized and specified air-diffusion technology to drive air towards low-level return paths
- Apply top-down air flow to minimise disruption from base-build ventilation systems instead of reliance on convection/plumes to transport contaminants
- Maintain sufficient velocity and air turbulence throughout the room to keep particulates buoyant and ensure their capture by HEPA filters
- Correctly position high-level air diffusers for consistent sweeping of the space
- Actively monitor occupancy and CO2 levels; ensure the ventilation rates are scaled to risk, reducing energy consumption
- Undertake downstream monitoring of airborne particulate levels, filter condition and ventilation rates for real-time reassurance that the system is protecting room users
- Consider other important comfort factors, such as attenuation of noise levels from fan/air
- Provide visual indications of system efficacy, reporting positive airflow and downstream particulate counts
- Colour code the air diffusers
About the authors:
Brendon McManus MCIBSE is CEO, Richard Fagg is lead designer and Steve Robinson is senior technical engineer all at Clean Air Technologies
References:
1 CIBSE Covid-19 Ventilation Guidance, v5 (July 2021)